
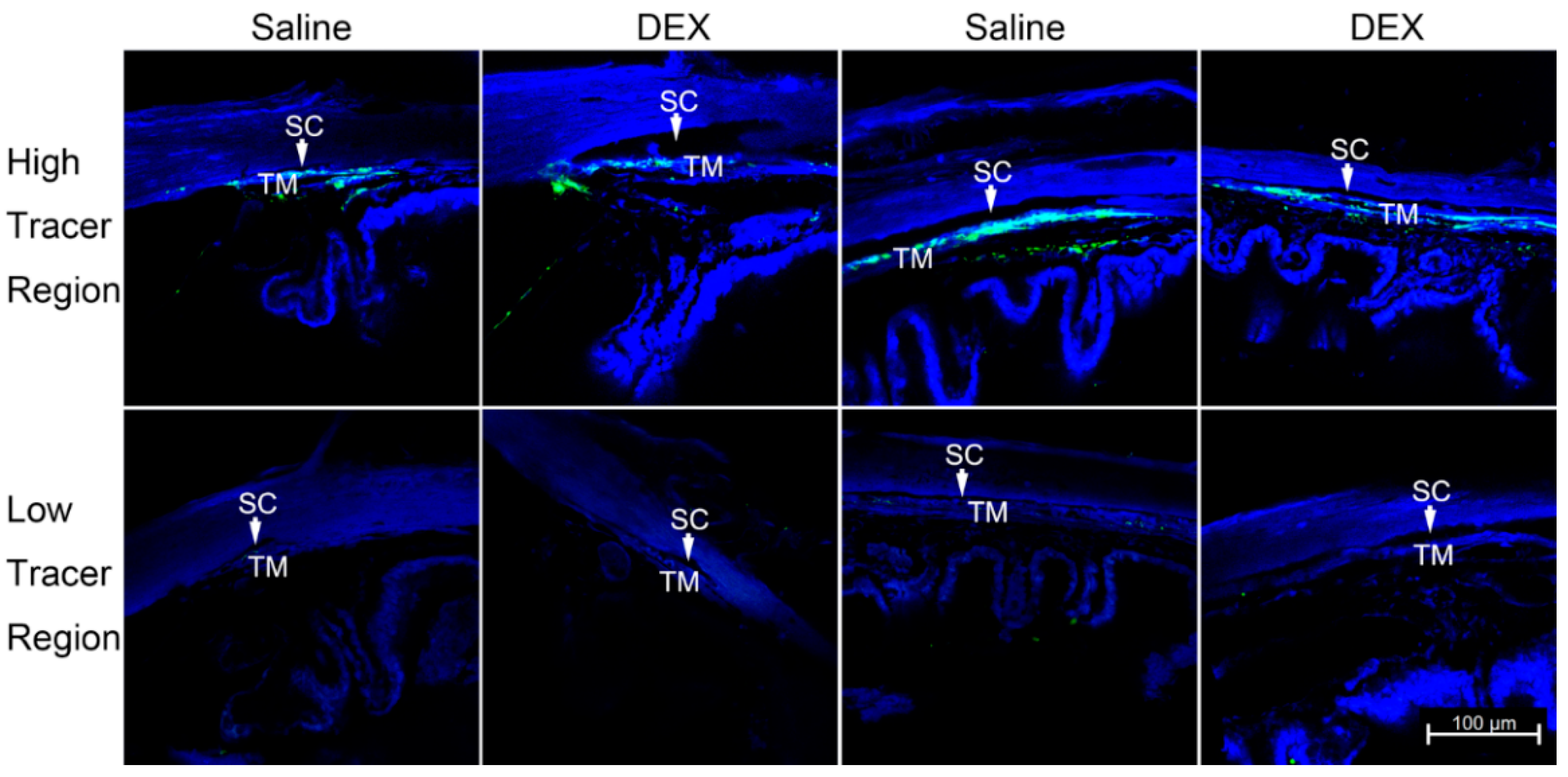
Notably, EA at the ST36 acupoint reversed these phenomena, indicating its curative effect in inflammatory pain.

The inflammatory pain model mice developed both mechanical and thermal hyperalgesia. We injected mice with carrageenan (carra) or a complete Freund’s adjuvant (CFA) to model inflammatory pain and investigated the analgesic effect of EA using animal behavior tests, immunostaining, Western blotting, and a whole-cell recording technique. TRPV1 (transient receptor potential vanilloid subtype 1) and TRPV4 are two crucial receptors involved in inflammatory pain, but their roles in EA- (electroacupuncture-) mediated analgesia are unknown. Furthermore, the herein proposed 3D co-culture platform that mimics the tumor stroma, is ideally suited to systematically investigate the factors influencing the penetration characteristics of newly developed nanomedicines to allow the design of nanoparticles with optimal penetration characteristics.Although pain is a major human affliction, our understanding of pain mechanisms is limited.
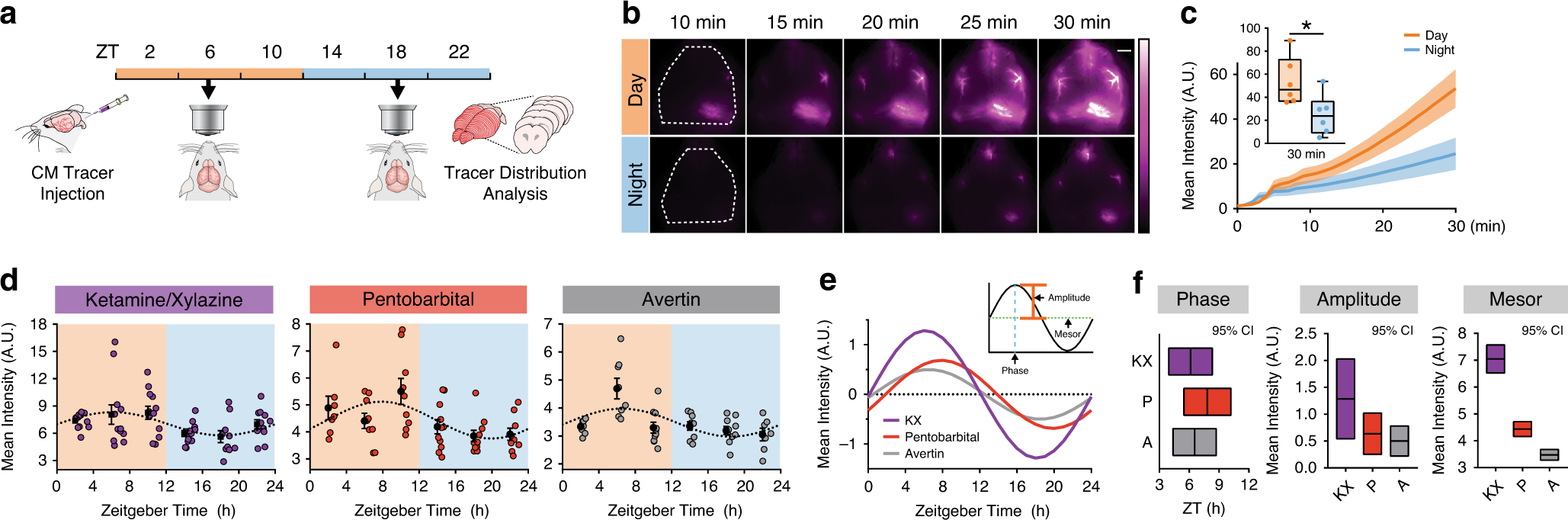
The 30-nm nanoparticles with low zeta potential favor deeper penetration. In conclusion, our data demonstrate that tumor stroma acts as a strong barrier for nanoparticle penetration. As a result, heterospheroids had significantly a lesser penetration of the nanoparticles compared to homospheroids. Furthermore, we also developed human homospheroids (MDA-MB-231 or Panc-1 tumor cells) and heterospheroids (MDA-MB-231/BJ-hTert and Panc-1/pancreatic stellate cells) and performed silica nanoparticle (30 and 100 nm) penetration studies. Subsequently, similar experiments were conducted using Cy5-conjugated pegylated PLGA nanoparticles and confocal laser scanning microscopy an increased nanoparticle penetration was found in 4T1 homospheroids until 48 h, but significantly lower penetration in heterospheroids. Silica nanoparticles with a less negative zeta potential exhibited lesser penetration compared to highly negative charged nanoparticles. In contrast, spheroids with increasing fibroblast amounts significantly inhibited NP penetration. Fluorescent microscopy on spheroid cryosections after incubation with silica nanoparticles showed that 4T1 homospheroids allowed a high penetration of about 75–80% within 24 h, with higher penetration in case of the 30 nm nanoparticles. Subsequently, we studied the penetration of high and low negatively charged fluorescent silica nanoparticles (30 nm red and 100 or 70 nm green zeta potential: − 40 mV and − 20 mV) and as well as Cy5-conjugated pegylated PLGA nanoparticles (200 nm, − 7 mV) in both homo- and heterospheroid models. Furthermore, immunohistochemical staining and gene expression analysis showed a proportional increase of α-SMA and collagen in heterospheroids with higher fibroblast ratios attaining 35% and 45% positive area at 5:1 (3T3:4T1) ratio, in a good match with the clinical breast tumor stroma.

Confocal live imaging revealed that fibroblasts distributed and reorganized within 48 h in heterospheroids. Next, we prepared homospheroids of 4T1 mouse breast cancer cells or 3T3 mouse fibroblasts alone as well as heterospheroids combining 3T3 and 4T1 cells in different ratios (1:1 and 5:1) using a microwell array platform. First, we examined human breast tumor patient biopsies to characterize the content and organization of stroma and found a high expression of alpha-smooth muscle actin (α-SMA 40% positive area) and collagen-1 (50% positive area). In the present study, we developed and thoroughly characterized a 3D co-culture spheroidal array to mimic tumor stroma and investigated the penetration of silica and PLGA nanoparticles in these spheroids. There is however a lack of suitable in vitro systems to study the tumor stroma penetration of nanoparticles. Most tumors possess abundant stroma, a fibrotic tissue composed of cancer-associated fibroblasts (CAFs) and extracellular matrix (ECM), which acts as a barrier for nanoparticle penetration. Nanoparticle penetration through tumor tissue after extravasation is considered as a key issue for tumor distribution and therapeutic effects.


 0 kommentar(er)
0 kommentar(er)
